Early and accurate diagnosis is critical in improving patient outcomes1
Variable dermatologic and hematologic presentation
*Study design: This retrospective analysis included 90 BPDCN patients (Male: 62, Female: 28; Age: 8-103 years) registered in the French Study Group on Cutaneous Lymphoma database between November 1995 and January 2012.7
Key clinical and pathological features of BPDCN12
Prior diagnoses should be considered
Approximately 10% to 20% of patients with BPDCN have a previous history of, or may have been misdiagnosed with, certain other hematologic malignancies, including MDS, CMML, and AML.1,12,13
Consider adding the CD123 marker to initial diagnostic panels1,14
CD123, in combination with other signature markers CD4 and CD56, is key in diagnosing BPDCN.1,16*
![]() Delay in the correct diagnosis can mean that by the time BPDCN is recognized, disease progression may have already occurred1,7,17
Delay in the correct diagnosis can mean that by the time BPDCN is recognized, disease progression may have already occurred1,7,17
*BPDCN can include other markers, such as TCL1, TCF4, and CD303 (BDCA2).1,18
Nodular lesions
Nodules are often purple/violet in color and localized to various body areas, particularly the trunk, limbs, and head.3,7
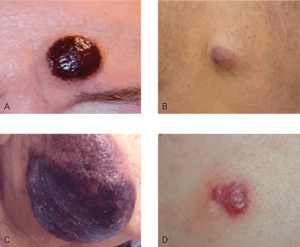
A. This is an electronic version of figures published in Br J Dermatol, September 2013, published by Wiley. B. This is an electronic version of figures published in Br J Dermatol, September 2013, published by Wiley. C. Courtesy of Shapiro R, et al. J Cell Sci Ther. S8:008. doi:10.4172/2157-7013.S8-008. D. Reprinted by permission from Springer Nature: Modern Pathology, Neoplasms derived from plasmacytoid dendritic cells, Facchetti F, © 2016.
Diffuse macules or hyperpigmented presentations
One or several macules with a rash or bruise-like appearance or hyperpigmented, red-brown macules found on various body areas.3,7,8
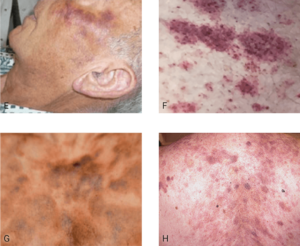
E. This is an electronic version of figures published in Br J Dermatol, published by Wiley. F. Republished with permission from American Society of Hematology. G. This is an electronic version of figures published in Br J Dermatol, published by Wiley. H. Reprinted with permission from Massachusetts Medical Society.
| Clinical features of cutaneous lesions12 | |
|
|
| Clinical features of cutaneous lesions12 | |
|
|
Be on the lookout for BPDCN—it may be more common than you think1-4
AML = acute myeloid leukemia; BPDCN = blastic plasmacytoid dendritic cell neoplasm; CMML = chronic myelomonocytic leukemia; MDS = myelodysplastic syndrome.
References: 1. Pagano L, et al. Blastic plasmacytoid dendritic cell neoplasm: diagnostic criteria and therapeutical approaches. Br J Haematol. 2016;174(2):188-202. 2. Laribi K, et al. Blastic plasmacytoid dendritic cell neoplasm: from origin of the cell to targeted therapies. Biol Blood Marrow Transplant. 2016;22(8):1357-1367. 3. Riaz W, et al. Blastic plasmacytoid dendritic cell neoplasm: update on molecular biology, diagnosis, and therapy. Cancer Control. 2014;21(4):279-289. 4. Goyal A, et al. Blastic plasmacytoid dendritic cell neoplasm. In: Carter JB, et al, eds. Atlas of Cutaneous Lymphomas: Classification and Differential Diagnosis. Cham, Switzerland: Springer International; 2015:193-203. 5. Pagano L, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica. 2013;98(2):239-246. 6. Pemmaraju N. Novel pathways and potential therapeutic strategies for blastic plasmacytoid dendritic cell neoplasm (BPDCN): CD123 and beyond. Curr Hematol Malig Rep. 2017;12(6):510-512. 7. Julia F, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169(3):579-586. 8. Sullivan JM, Rizzieri DA. Treatment of blastic plasmacytoid dendritic cell neoplasm. Hematology Am Soc Hematol Educ Program. 2016(1):16-23. 9. Shapiro R, et al. Blastic plasmacytoid dendritic cell neoplasm: a review of diagnosis, pathology, and therapy. J Cell Sci Ther. 2015;S8:008. doi:10.4172/2157-7013.S8-008. 10. Wong C, et al. Dermatopathologists’ opinions about the quality of clinical information in the skin biopsy requisition form and the skin biopsy care process: a semiqualitative assessment. Am J Clin Pathol. 2015;143(4):593-597. 11. Herling M, Jones D. CD4+/CD56+ Hematodermic tumor: the features of an evolving entity and its relationship to dendritic cells. Am J Clin Pathol. 2007;127:687-700. 12. Reichard KK. Blastic plasmacytoid dendritic cell neoplasm: how do you distinguish it from acute myeloid leukemia? Surg Pathol Clin. 2013;6(4):743-747. 13. Blastic plasmacytoid dendritic cell neoplasm. https://seer.cancer.gov/seertools/hemelymph/51f6cf56e3e27c3994bd530c/. Accessed April 8, 2025. 14. Facchetti F, et al. Neoplasms derived from plasmacytoid dendritic cells. Mod Pathol. 2016;29(2):98-111. 15. Vitte F, et al. Specific skin lesions in chronic myelomonocytic leukemia: a spectrum of myelomonocytic and dendritic cell proliferations: a study of 42 cases. Am J Surg Pathol. 2012;36(9):1302-1316. 16. Pemmaraju N, Konopleva M. Treating blastic plasmacytoid dendritic cell neoplasm. The Hematologist website. https://ashpublications.org/thehematologist/article/doi/10.1182/hem.V15.5.8927/462977/Treating-Blastic-Plasmacytoid-Dendritic-Cell. Published August 28, 2018. Accessed April 8, 2025. 17. Frankel AE, et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood. 2014;124(3):385-392. 18. Ceribelli, et al. A druggable TCF4- and BRD4-dependent transcriptional network sustains malignancy in blastic plasmacytoid dendritic cell neoplasm. Cancer Cell. 2016;30(5):764-778.